After the coronavirus pandemic taking a toll on India with minimal testing facilities, several labs all across India applied to the Pune-based National Institute of Virology to get their testing kits vetted. However, MyLab from Pune is the very first manufacturer to receive approval from the institute for the deployment of the COVID-19 testing kits.
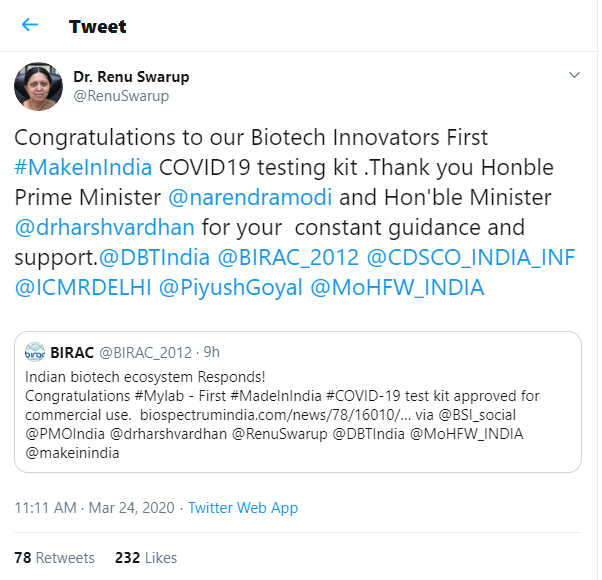
Renu Swarup, the Secretary of the Department of Biotechnology tweeted a congratulatory message to the company’s biotech innovators for the very first COVID-19 testing kit that is completely under the Make-in-India initiative. Miss Swarup is also the lead at BIRAC that extends support to the biotech companies & MyLab was among them.
The COVID-19 diagnostic kits are currently massively reliant on foreign companies. This is why several companies throughout India applied for the country’s very own diagnostic kit. As per the guidelines posted by the ICMR, the diagnostic kits duly approved by the U.S. FDA or the ones certified by the EU shall be used for the detection of SARS CoV-2 at a commercial level.
However, on Monday, Dr Balram Bhargava, the Director-General at ICMR stated that wasn’t necessary anymore. The ones that have been approved by the National Institute of Virology shall be eligible for use.
 Amazing India Blog Know India Better
Amazing India Blog Know India Better